History
Headquartered in Halmstad in the South of Sweden, Redsense Medical traces back to the year 2000, after a number of incidents with venous needle dislodgement during Hemodialysis in Halmstad County Hospital.
The nurses came to the realization that these incidents were far from unique and that existing alarm systems were widely notorious for malfunctioning. The hospital technicians contacted engineer Daniel Engvall, co- founder of Redsense, looking for a solution to this problem.
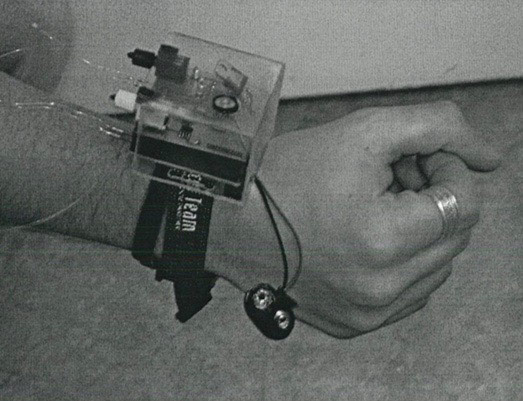
Daniel started a project with the goal of developing the first monitoring system for venous needle dislodgement during hemodialysis. The project was supported by Innovation Team, a well-established design company in the medical technology sector, and together with the local company a first prototype was developed.
In late 2003 the development of the first product prototype was finalized and in 2004 a patent protecting its basic technology was filed. During the development process, the team evaluated existing methods and, instead of attempting to measure activities inside the body or the dialysis equipment, designed a device which keeps an eye on the access points and monitors for the first sign of blood loss. An absorbent patch, which contains a blood sensor, is placed directly over the venous needle blood access point to absorb the blood that flows out from the patient in a situation where venous needle dislodgement occurs. The sensor inside the patch is connected to an external alarm unit to allow the sensor patch to be as small and simple as possible.
In 2006 Patrik Byhmer, board member of Innovation Team, came across the project and founded the company Redsense Medical AB as a spin-off of the project, together with Daniel Envall and help from investors.
Timeline
2021
- Smart wound care technology in spin-off company “Odinwell AB”
2020
- Directed new share issue of 1,5 million shares, raising Sek 73 Million
- All top 5 U.S. Dialysis providers and 3 of the top 5 best U.S. nephrology hospitals now use Redsense
2019
- Home hemodialysis machine manufacturer Physidia commits to integrate Redsense through PAS63023
- Listing change from NASDAQ First Noth to Swedish “Spotlight” Stock Market
- Innovative technology for smart bandages patent approval
- CVC sensor patch technology patent approval
2018
- Redsense expands operations into new market with innovative technology for smart bandages
- Redsense distributor for Italy was one of the winners of CONSIP tender using Redsense in connection to dialysis machine through PAS63023
- Evaluation with large dialysis provider in the US for 15000 treatments over 26 clinics
- Successful Study with Clamp prototype at UHN Toronto, Canada, lead by Dr. Christopher Chan
- 3 of 5 largest dialysis providers in the US, and 3 of 5 largest dialysis clinics in the US, use Redsense to monitor dialysis treatments
2017
- Listing change from Swedish Stock Market Aktietorget to NASDAQ First North
- Successful usability study of catheter sensor at Vivantes Klinikum Berlin, Germany
- Redsense catheter sensor launched in the US
2016
- Governmental decision to increase hemodialysis in Czech Rep. – cooperation with B.Braun in Czech Rep. – also in Slovakia
- Villingen-Schewnningen as first German user of Redsense for home hemodialysis
- The International Electrotechnical Commission’s (IEC) committee for dialysis has clearly stated in the standard for dialysis equipment (IEC 60601-2-16) that the pressure alarm isn’t sufficient to detect VND – PAS63023 was released, which allows a dialysis machine to communicate with an external alarm. Nikkiso DBB-EXA machine integrates Redsense bloodloss alarm this way.
- Redsense CVC patch for catheter approved for use in Europe and CE marked – FDA application and usability studies in process
2015
- NxStage receives clearance for at-home nocturnal dialysis in the US, with Redsense written on the label. Launch starts in February 2015
- Listing on the Swedish “Aktie Torget” Stock Market
2014
- Production of the 2nd generation Redsense starts
- Launch of the new generation begins in April to existing customers in the US, UK and the Nordic countries.
- Together with a supplier, Redsense Medical receives funding from the EuroStars project (an EU project for innovations by European companies)
2013
- CE marking of the 2nd generation Redsense, i.e. the product is approved for sales in Europe
- Commercial availability of the 2nd generation Redsense in the US, i.e. the product is approved for sales in the US
2012
- A license and financing agreement is struck with a company in the industry (NxStage Medical Inc., which is listed on Nasdaq)
2007–2011
- Becomes CE marked and thus approved for sale in Europe
- First order from the Royal Western Infirmary Hospital in Glasgow, Scotland
- Receives FDA 510k clearance (2007), in other words, approved for sales in the US. Centers for Medicare & Medicaid Services confirm that the Redsense product will be covered by Medicare
- Redsense Medical AB receives ISO13485 certificate
- FDA 510k clearance for Home dialysis » US Government of Veteran A airs (VA) makes the use of Redsense for high-risk patients mandatory
- FDA 510k clearance for nocturnal treatment in clinic (2010)
- The Redsense Medical group is established through the foundation of the wholly-owned subsidiary Redsense Malta and Redsense Medical Inc. in the USA
2006
- Redsense Medical AB was established
- Clinical testing at five Swedish hospitals